7 rows · However, guanidine has a pKa of 125(3) and should exist almost entirely as a cation underPH 74 • Elution Buffer PBS with 6M guanidine•HCl and 250mM imidazole;PH 74 For resin regeneration prepare the following buffer • MES Buffer mM 2(Nmorpholine)ethanesulfonic acid, 01M sodium chloride;

Solved Solutions Fv 15ml Gte 50mm Glucose 25mm Tris Ph Chegg Com
Guanidine hcl ph
Guanidine hcl ph-MCLAB's 7M GuanidineHCl Solution is a readytouse solution of guanidine hydrochloride, which can be easily diluted and pHadjusted to any concentration below 7M In addition to increasing solubility of hydrophobic molecules, guanidine is a general protein denaturant, unfolding proteins and altering their structuresThe inclusion bodies were solubilized with 8M urea or 6M guanidinehydrochloride at pH 74, and the recombinant protein was purified by NiNTA column The purified fusion protein was refolded by dialysis with a gradient of decreasing concentration of urea or guanidine hydrochloride or by the size exclusion protein refolding system


Guanidine Hydrochloride Guanidine Hcl Cas 50 01 1 Yacooscience Com
Guanidine hydrochloride is commercially available from Sigma Aldrich The MW is 955 therefore one mole weight 955 g If you want to prepare one liter of solution you need 8 x 955 g of this saltGuanidine Hydrochloride is a strong protein denaturant that functions as a chaotropic agent As a denaturant, it acts to unfold proteins and turn them into their original polypeptide chains As a chaotropic agent, it breaks down the structure of proteinsGuanidine Hydrochloride, BiotechGrade is considered a chaotrope and a strong denaturants utilized in physiochemical studies of protein folding Proteins become randomly coiled at high concentrations of guanidinium chloride Spectrum offers highly pure reagents suitable for biochemical research and analysis
Polyhexamethylene guanidine hydrochloride is a kind of colorless or light yellow solid or liquid It is soluble in water and free of erosion PHMG biocide is environmentalfriendly and free from iodine, chlorine, aldehyde, and alcoholCyanuric Acid (CIA) Magnesium Chloride;Oct 15, · Aqueous solutions with a concentration of % have a pH of 135 at 25 °C If an aqueous solution of guanidine is heated, guanidine will hydrolyze to urea As a base, guanidine will react with acids to form salts By protanating guanidine, the guanidinium ion is formed HNC(NH 2) 2 HCl → C(NH 2) 3 Cl
Guanidine Isothiocyanate Solution 4 M guanidine isothiocyanate, 50 mM TrisHCl (pH 75), 25 mM EDTA Cat No Size 500 ml Store at 4°C Description Guanidine isothiocyanate is a strong protein denaturant In a highly concentrated guanidine isothiocyanateGuanidine hydrochloride solution 8 M, pH 85, buffered aqueous solution NACRES N5Its hydrochloride salt is highly watersoluble and is the usual article of commerce The free base is extremely toxic, as shown in the hazard information table Guanidine was discovered in nature in the late 19th century In 1907, a German patent was awarded to Italian chemist Celso Ulpiani for the reaction of dicyanamide with strong acid to



Guanidine Hcl


Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Elife
55 M guanidine thiocyanate (GuSCN) mM Tris HCl pH 66;PH 4060 Specific Gravity/Density g/cm3 Vapor Pressure Negligible Solubility 573 g/L at oC Vapor Density Not available 2 Molecular Formula NH C(NH)NH 2 ·HCl Viscosity Not available Molecular Weight 9553 g/molMolecular Formula CH 5 N 3 • HCl Solubility in Water (g/L) 573 FW 9553 g/mol pH @ °C 45 60 pKa @ ˚C 136 Guanidine Hydrochloride 6M solution can be used to denature proteins which become randomly coiled with no residual structure



Ph Corrections And Protein Ionization In Water Guanidinium Chloride Sciencedirect


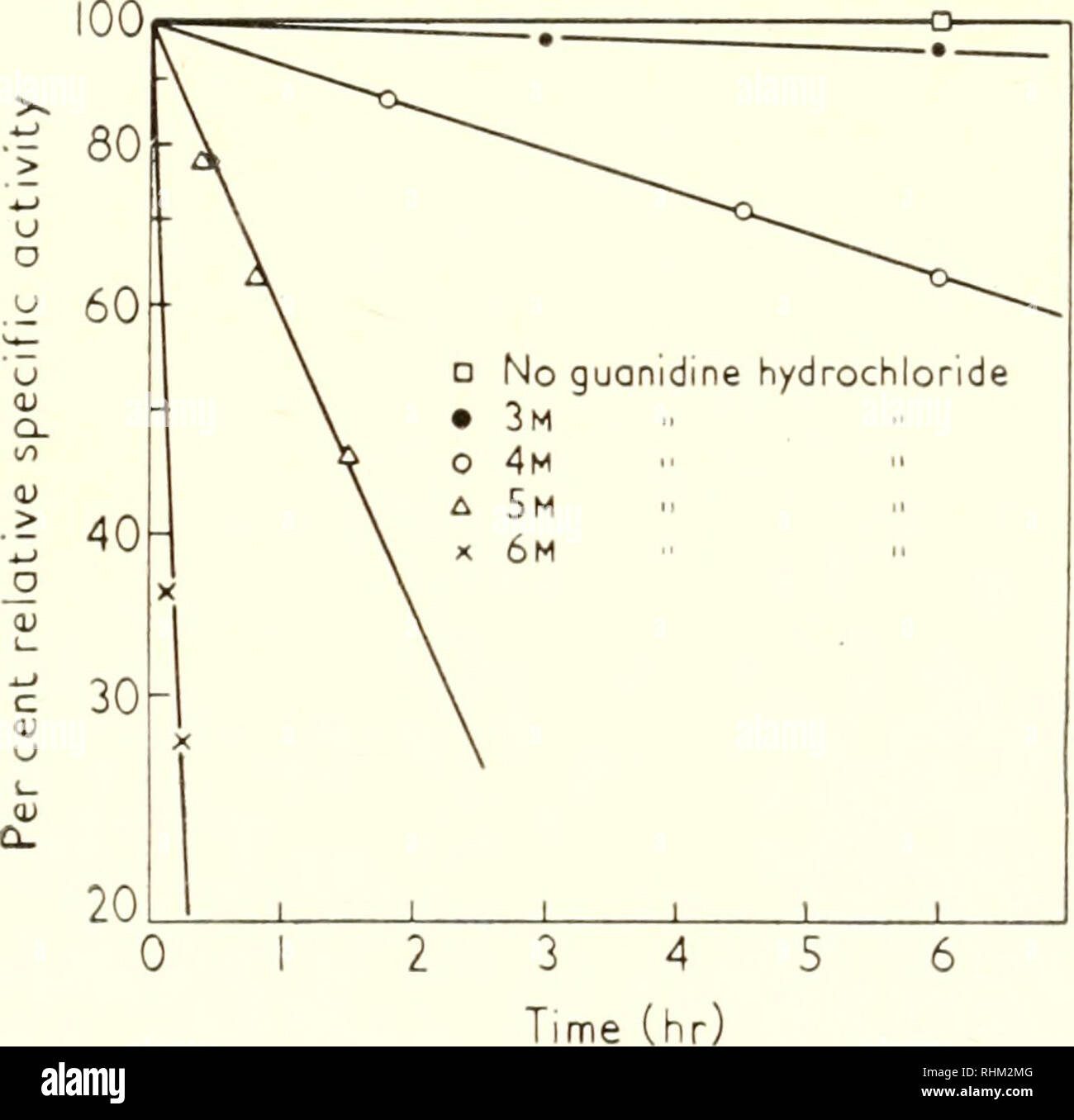
Biological Structure And Function Proceedings Biochemistry Cytology 64 Gertrude E Perlmann Table Iii Rotatory Dispersion Constant A And Specific Activity Of Pepsin In Various Solvents As Function Of Temperature Relative Specific
50 mM MOPS pHPrepare of 6 M guanidine hydrochloride by dissolving of guanidine hydrochloride into of dH 2 O Step 2 Once dissolved, complete the volume to with dH 2 OBuffer QX1 (for solution and binding of agarose gels) 7 M NaPO4;



Table I From The Effect Of Guanidine Hydrochloride On Crystalline Pepsin Semantic Scholar



Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Biorxiv
Guanidine Hydrochloride is the hydrochloride salt form of guanidine, a strong basic compound with parasympathomimetic activity Guanidine hydrochloride enhances the release of acetylcholine following a nerve impulse and potentiates acetylcholine actions on muscarinic and nicotinic receptors It also appears to slow the rates of depolarization and repolarization of muscle cellBuffer PE 10 mM TrisHCl pH 75;• Wash Buffer PBS with 6M guanidine•HCl and 25mM imidazole;



Guanidinium Thiocyanate Wikipedia


8m Guanidine Hcl Solution
Dec 01, 01 · More than 30 years ago, Nozaki and Tanford reported that the pK values for several amino acids and simple substances in 6 M guanidinium chloride differed little from the corresponding values in low salt (Nozaki, Y, and C Tanford 1967J Am Chem Soc 736–742) This puzzling and counterintuitive result hinders attempts to understand and predict the protonQG (QIAGEN cat# , 250ml) 55 M guanidine thiocyanate (GuSCN), mM TrisHCl, pH 66 (25ºC), dissolve in pH 7 standard solution or water AE (elution buffer for genomic DNA preps) 10 mM TrisHCl, pH 80 05 mM EDTA, pH90 QX1 (solubilization and binding of agarose gels) (QIAGEN cat# 912, 500ml) 7M NaPO4 10mM NaAc, pH 53How to Run guanidine hydrochloride sample in SDS gel ?



Powder Guanidine Hcl 25 Kg Rs 100 Kilogram A B Enterprises Id


Guanidine Banque D Image Et Photos Alamy
Oct 03, 17 · Alternate Names Guanidine Hydrochloride is also known as Aminoformamidine Hydrochloride Application Guanidine Hydrochloride is a widely used and powerful protein denaturant used in the purification of proteins and nucleic acids CAS Number Purity ≥99% Molecular Weight 9553GUANIDINE HCL, 80M SOLUTION Safety Data Sheet according to Federal Register / Vol 77, No 58 / Monday, March 26, 12 / Rules and Regulations 02/18/15 EN (English US) 2/6 Name Product identifier % Classification (GHSUS) guanidine,hydrochloride (CAS No) 70 80 Acute Tox 4 (Oral), H302 Skin Irrit 2, H315Virus inactivation of plasmaderived proteins by pasteurization in the presence of guanidine hydrochloride Transfusion 01 Mar;41(3)39 doi /jx Authors A Schlegel 1 , A Immelmann, C Kempf Affiliation 1 ZLB Bioplasma AG, Bern



His Select Cobalt Affinity Gel H8162 Technical Sigma Aldrich
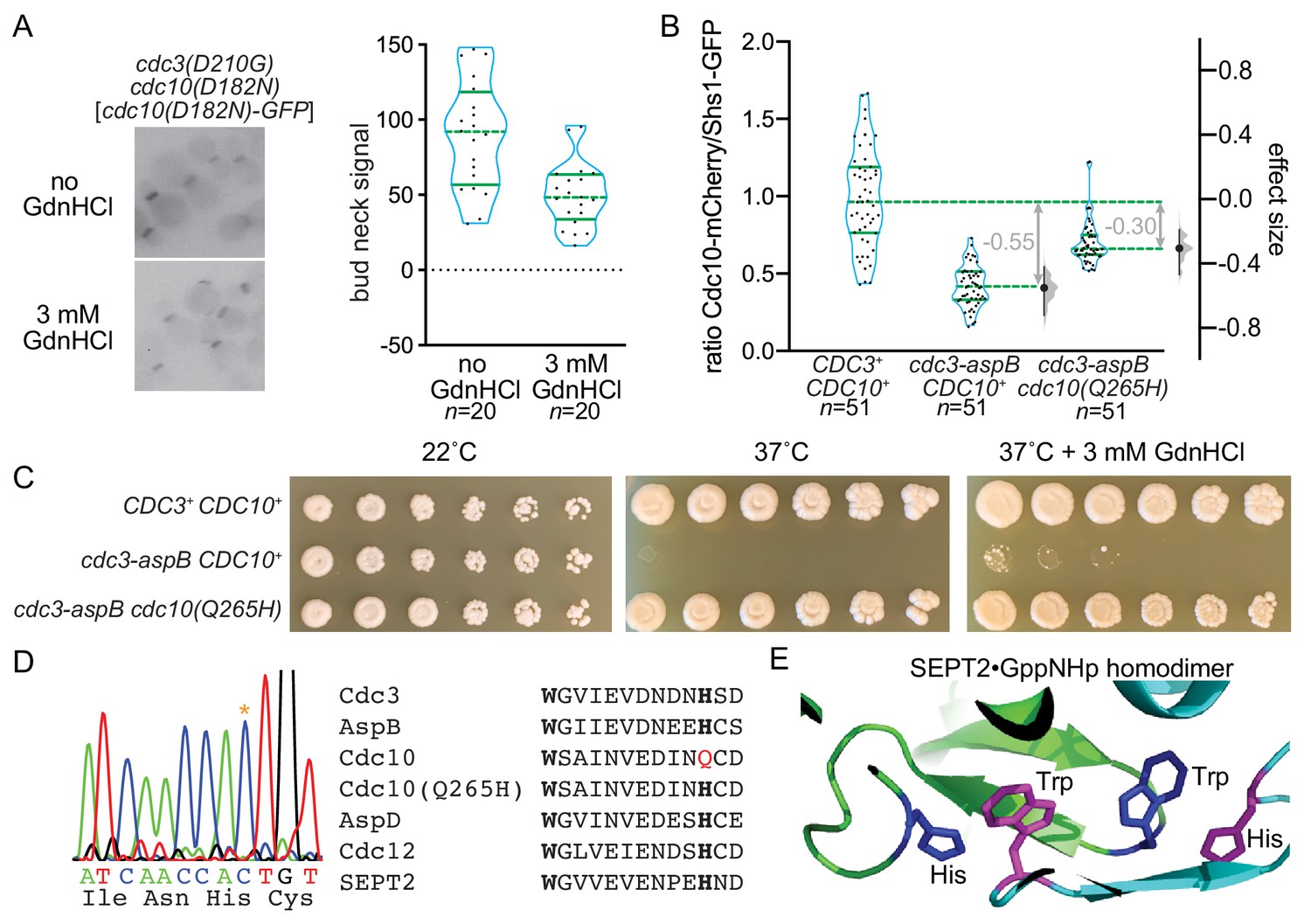


Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Elife
Strong chaotropic agent useful for the denaturation and subsequent refolding of protein Used in the isolation of RNA Solubilizes insoluble or denatured proteins such as inclusion bodies Can be used for the recovery of periplasmic proteinsFind patient medical information for guanidine oral on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratingsDescription Thermo Scientific Pierce GuanidineHCl can be easily dissolved and pHadjusted to any concentration Features of GuanidineHCl • No wasting valuable research time weighing and dissolving guanidine crystals • Free from UV absorbing materials in the range of nm • Free from heavy metal contaminants


Guanidine Hydrochloride Manufacturers China Guanidine Hydrochloride Factory Suppliers



Guanidine Hydrochloride 50 01 1 Wiki
Description Thermo Scientific Pierce 8M GuanidineHCl Solution is an accurately formulated, readytouse solution of guanidine hydrochloride, that can be easily diluted and pHadjusted to any concentration below 8M Features of 8M GuanidineHCl Solution • Any concentration below 8M guanidine can be quickly and easily preparedGuanidine Hydrochloride is a strong proteindenaturing agent used in the isolation of RNA from cell extracts RNase activity is inhibited in the presence of this reagent SpecificationsGuanidine hydrochloride, CH 5 N 3HCL;
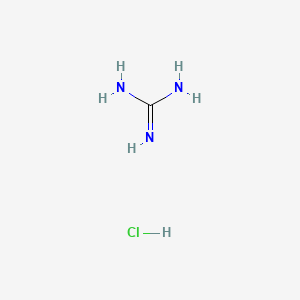


Guanidine Hydrochloride Ch5n3 Clh Pubchem


Imidacloprid Guanidine Hydrochloride C9h12cl2n4 1272 53 3
Aug 08, 14 · Aggregation of hen or human lysozyme depends on certain conditions, namely acidic pH or the presence of additives In the present study, the effects on the aggregation of hen eggwhite lysozyme via incubation in concentrated solutions of three different chaotropic agents namely guanidine thiocyanate, guanidine hydrochloride and urea wereThe US Army used guanidine hydrochloride as a surrogate for guanidine in toxicological testing conducted in the 1980s Results are presented for the two compounds as a group Results A Description and uses of guanidine and guanidine hydrochloride Guanidine is a strong organic base that is found in the urine as aThis substance is used in the following products laboratory chemicals, pH regulators and water treatment products, polymers, pharmaceuticals, photochemicals and extraction agents GuanidineHCl C&L Inventory guanidine;hydrochloride Other Guanidinhydrochlorid Registration dossier Guanidinium chloride C&L Inventory



Figure 1 From Entropic Stabilization Of Myoglobin By Subdenaturing Concentrations Of Guanidine Hydrochloride Semantic Scholar


Chemidplus 50 01 1 Pjjjbbjscakjqf Uhfffaoysa N Guanidine Hydrochloride Similar Structures Search Synonyms Formulas Resource Links And Other Chemical Information
Buffer QXB (for binding of large >3000 bp fragments to columns) 5 M GuHCl;Question 14 answers Asked 7th Aug, 18 In the latter case, there is no garantee that 005 M solution of TrisHCl would have a pH8 Consider solubilizing the sample in 5M guanidine HCl, and then use a far more dilute concentration of guanidine HCl in the SEC eluent 9 The pH of the mobile phase with 5M guanidine HCl may be near neutral (you need to measure pH) 10 If the mobile phase pH is near 70, guanidine HCl is more of a salt (chloride counter ion)



A New Approach To Study The Physical Stability Of Monoclonal Antibody Formulations Dilution From A Denaturant Journal Of Pharmaceutical Sciences



The Effect Of Guanidine Hydrochloride On Phase Diagram Of Peg Phosphate Aqueous Two Phase System Semantic Scholar
Guanidine hydrochloride has also typically been used for the isolation of RNA, to denature globular proteins, and for protein refolding studies It can also be used to facilitate the generation of tryptic peptides for analysis of complex protein samples Physical form This product is a readytouse 6 M guanidine hydrochloride solutionPH to desired pH using 10 M HCl or 10 M NaOH B Make Gua·HCl/peptide solution (Gua·HCl ~ 7M, 5 µM peptide, 5 mM K P Phosphate) 1 Purge Gua·HCl stock solution with argon 2 Mix the following solutions 147 mL Gua·HCl stock solution 150 µL 500 mM K P Phosphate (pH 80) 150 µL peptide solution 3 pH to desired pH using 10 M HCl or 10 M NaOHBuffer QBT (equilibration buffer) 750 mM NaCl;



Unfolding Of Bprp Wild Type And E211k Variant In Guanidine Download Scientific Diagram



1h Pyrazole 3 Carboxylic Acid 4 5 Dihydro 5 Oxo 1 4 Sulfophenyl 4 4 Sulfophenyl Azo Reaction Products With Guanidine Hydrochloride N N Bis Mixed Ph Tolyl And Xylyl Derivs 14 0 Wiki
Guanidine HCl at millimolar concentrations, is able to causes efficient loss of the normally stable PSI element from yeast cells 5 mM Guanidine HCl in growth media cures PSI and other prions of yeast 5 mM Guanidine HCl significantly reduces Hsp104mediated basal and acquired thermotolerance by 30fold and 50 fold, respectivelySulfamic Acid, NH 2 SO 3 H;Night at 4†Ž, pH of this solution was adjusted to 70 and then the solution was diluted with deionized water The above solution was mixed with an equal volume of 02M sodium chloride solution contg denaturing reagent for titrimetric assay, or with an equal volume of 5/M TrisHCl buffer (pH 70) contg



Usa1 Method For Rapid Isolation Of Rna And A Kit Thereof Google Patents


Extraction Kits How Do Kits Work On Dna
Guanidine HCl is 28 times more effective than urea in unfolding ribonuclease but only 17 times more effective for lysozyme G H2Oapp values of Guanidine HCl are 97 Cal/mole for ribonuclease at pH 66, 61 for lysozyme at pH 29, for αchymotrypsin at pH 43, and 117 for βDescription Thermo Scientific™ Pierce 8M GuanidineHCl Solution is an accurately formulated, readytouse solution of guanidine hydrochloride, that can be easily diluted and pHadjusted to any concentration below 8MJun 01, 09 · We have developed a guanidine hydrochloride (GnHCl)based stripping solution (6 M GnHCl, 02% Nonidet P40 NP40, 01 M βmercaptoethanol, and mM Tris–HCl, pH 75) that can rapidly dissociate antibodies from immunoblots at room temperature without removing significant amounts of the transferred proteins



Protein Stiffening And Entropic Stabilization In The Subdenaturing Limit Of Guanidine Hydrochloride Biophysical Journal



Effect Of Peg Salt W W Ratio And Ph On Partition Coefficient Of Download Scientific Diagram
Liquid Guanidine Hydrochloride, CH 5 N 3HCL;Dissolve the target protein in 6M guanidine HCl (or 8M urea), 50mM TrisHCl (pH 8), 2–5mM DTT Heat at 37°C for 45–60 minutes For denatured proteins, add 50mM NH 4 HCO 3 or 50mM TrisHCl (pH 78), 1mM CaCl 2 , until the guanidine HCl or urea concentration is less than 1MEthanol, like Guanidine HCL, is a chaotopic agent Ethanol is added for 2 reasons Enhance and influence the binding of nucleic acids to the silica Interfere with noncovalent intramolecular forces as outlined above Reduce association of nucleic acids with water From Melzak et al (1996)


1h Pyrazole 3 Carboxylic Acid 4 5 Dihydro 5 Oxo 1 4 Sulfophenyl 4 4 Sulfophenyl Azo Reaction Products With Guanidine Hydrochloride N N Bis Mixed Ph Tolyl And Xylyl Derivs 14 0
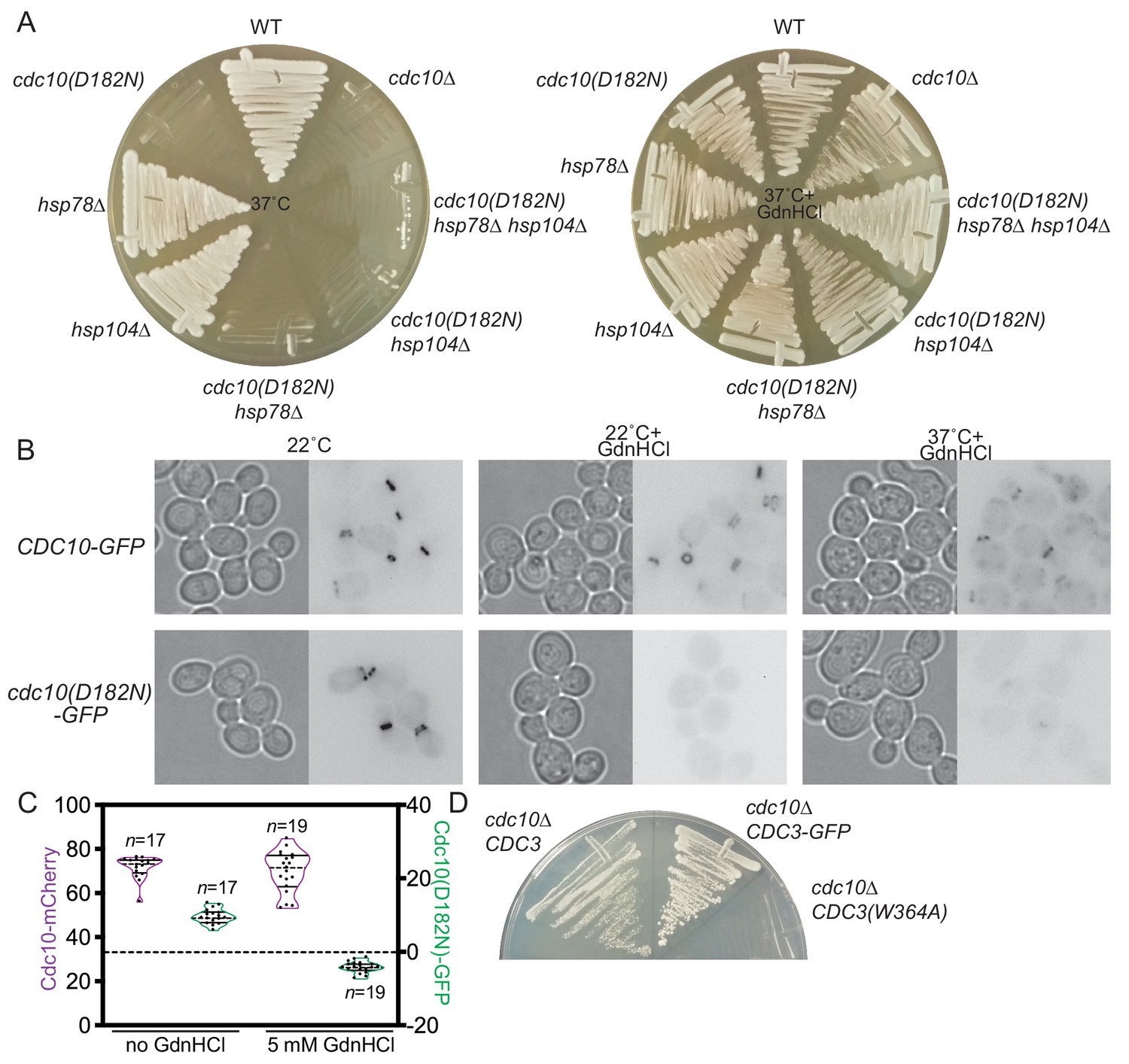


Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Elife
HCl final pH 75;Guanadine HCL is one of the strongest denaturing agents for protein folding studies, and is used to denature proteins due to its chaotropic qualitiesAmmonium sulfamate, NH 2 SO 3 NH 4;



Guanidine Hydrochloride Guanidine Hcl Cas 50 01 1 Yacooscience Com



Preparation And Extraction Of Insoluble Inclusion Body Proteins From Escherichia Coli Abstract Europe Pmc
Magnesium Sulfate Heptahydrate, MgSO 47H 2 O;Denaturing buffer containing guanidine hydrochloride Next Section 6 M GuanidineHCl 5 mM DTT 50 mM TrisCl (pH 75) Previous Section Warmm the solution to 40ºC to dissolve the guanidine HClAdjust the pH to 40 using 1M NaOH or 1M HCl Bring the volume to 100 ml using deionised water and filter sterilize the buffer using a 045 µm filter Safety Urea and guanidine hydrochloride are both skin and eye irritants



Assisting The Reactivation Of Guanidine Hydrochloride Denatured Aminoacylase By Hydroxypropyl Cyclodextrins Biophysical Journal



Unfolding Of Huprp 90 231 In Guanidine Hydrochloride At Ph 7 2 A Download Scientific Diagram



Guanidine For Molecular Biology 99 50 01 1 Sigma Aldrich



Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Biorxiv
-500x500.jpg)


Streptavidin Elution Buffer



Figure 4 From Avidin 4 Stability At Extremes Of Ph And Dissociation Into Sub Units By Guanidine Hydrochloride Semantic Scholar



Guanidine Hydrochloride Bio Basic Bio Basic
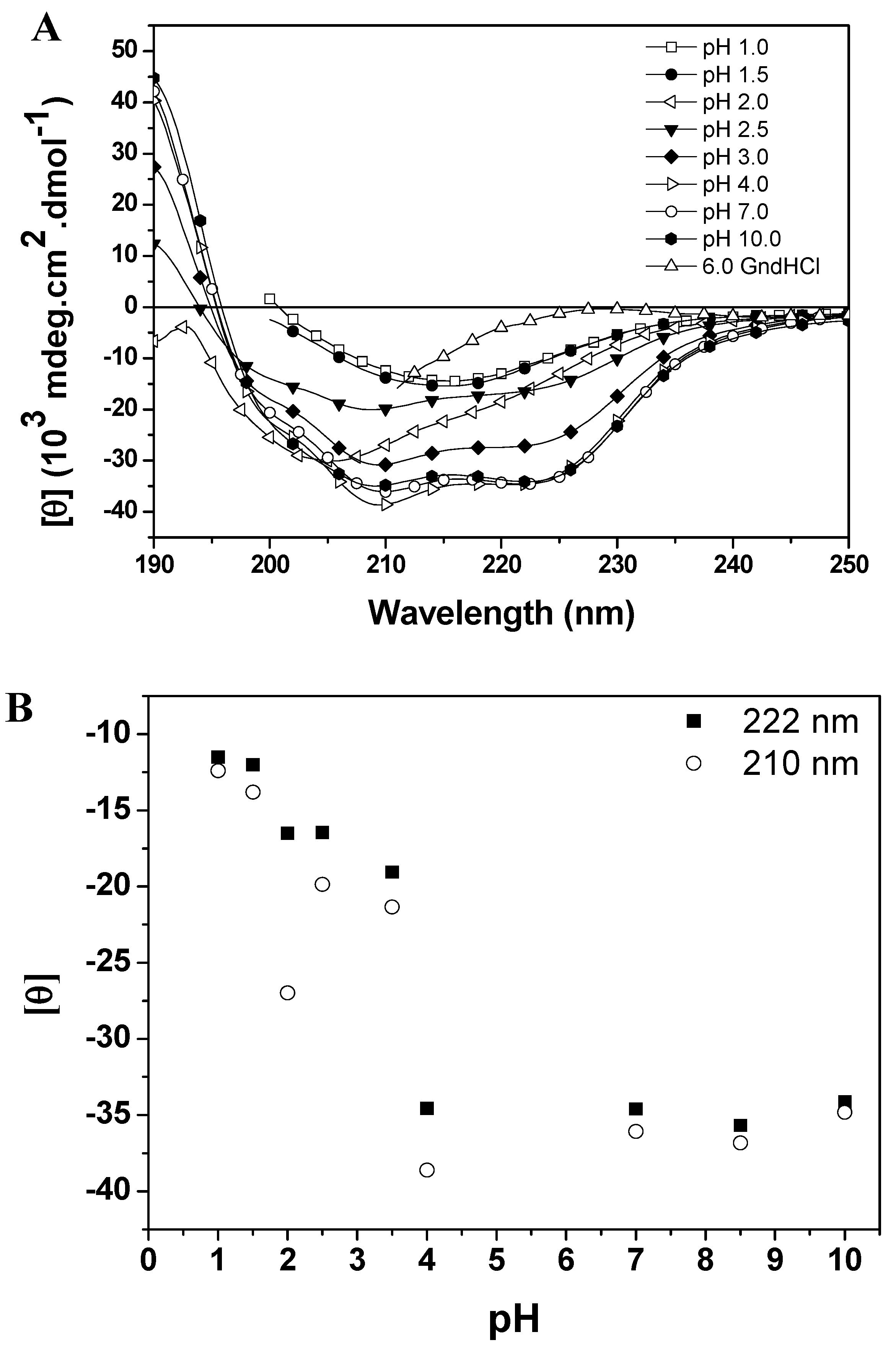


Molecules Free Full Text Unfolding Studies Of The Cysteine Protease Baupain A Papain Like Enzyme From Leaves Of Bauhinia Forficata Effect Of Ph Guanidine Hydrochloride And Temperature Html



Should I Use Guanidine Hcl B2619a In Colorimetric Lamp Reactions Neb



Solved Solutions Fv 15ml Gte 50mm Glucose 25mm Tris Ph Chegg Com


Molecules Free Full Text Unfolding Studies Of The Cysteine Protease Baupain A Papain Like Enzyme From Leaves Of Bauhinia Forficata Effect Of Ph Guanidine Hydrochloride And Temperature Html


Plos One Differences In The Pathways Of Proteins Unfolding Induced By Urea And Guanidine Hydrochloride Molten Globule State And Aggregates



Molecules Free Full Text Unfolding Studies Of The Cysteine Protease Baupain A Papain Like Enzyme From Leaves Of Bauhinia Forficata Effect Of Ph Guanidine Hydrochloride And Temperature Html


Denaturation Of Proteins In Guanidine Hcl Fluorescence Of 0 3 M Download Scientific Diagram



Liquid Liquid Equilibria Electrical Conductivity And Refractive Indices Of Poly Ethylene Glycol Sodium Sulfate Guanidine Hydrochloride Aqueous Two Phase Systems Correlation And Thermodynamic Modeling Sciencedirect



8m Guanidine Hcl Solution



Guanidine Hydrochloride 99 Cas 50 01 1 Glentham Life Sciences
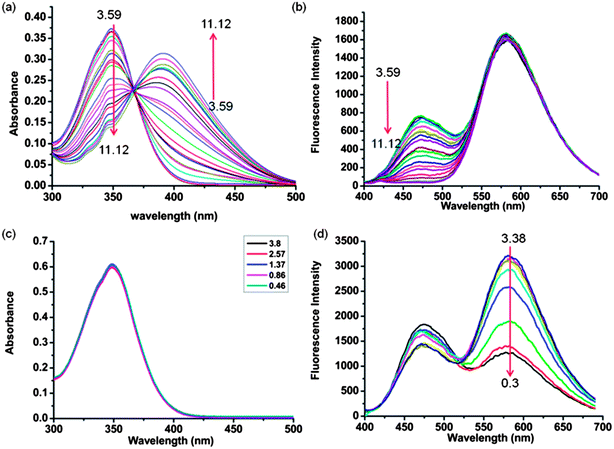


A Guanidine Derivative Of Naphthalimide With Excited State Deprotonation Coupled Intramolecular Charge Transfer Properties And Its Application Journal Of Materials Chemistry C Rsc Publishing



4 Chlorophenyl Guanidine Hydrochloride Lgc Standards



Guanidine An Overview Sciencedirect Topics


Plos One Search For Independent B A 4 Subdomains In A B A 8 Barrel B Glucosidase



Guanidine Hydrochloride Solution 8 M Ph 8 5 Buffered Aqueous Solution Sigma Aldrich



Guanidine Hydrochloride Induced Unfolding Transition Curve As Measured Download Scientific Diagram



Potassium Acetate 3m Ph 5 5 Cepham Life Sciences Research Products



Entrapping Intermediates Of Thermal Aggregation In A Helical Proteins With Low Concentration Of Guanidine Hydrochloride Journal Of Biological Chemistry


Acidic P H And Detergents Enhance In Vitro
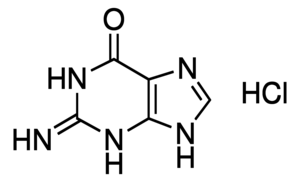


Guanidine For Molecular Biology 99 50 01 1 Sigma Aldrich



Guanidine 50 01 1 Sigma Aldrich



Isothermal Chemical Denaturation As A Complementary Tool To Overcome Limitations Of Thermal Differential Scanning Fluorimetry In Predicting Physical Stability Of Protein Formulations Sciencedirect


Biological Structure And Function Proceedings Biochemistry Cytology The Relation Of The Secondary Structure Of Pepsin 6i Readily Inactivated If Brought Into Contact With These Reagents Pepsin Remains Active After Short Exposure


Guanidine Hydrochloride 5 Kg Reagents For Protein Isolation Protein Isolation Biochemistry Life Science Carl Roth International



Unfolding Of Cutinase At Ph 4 5 Induced By Guanidine Hydrochloride Download Scientific Diagram



Guanidine Hydrochloride Solution 6m Molecular Depot



Acros Organics Ac Guanidine Hydrochloride 98 1kg Cas 50 01 1 From Cole Parmer Canada



Potassium Acetate Guanidine Hydrochloride Solution Ph 4 2 32 41 Bioworld



Guanidine Hcl



Figure 1 From Evidence For Residual Structure In Acid And Heat Denatured Proteins Semantic Scholar



Unfolding Of Cutinase At Ph 4 5 Induced By Guanidine Hydrochloride Download Scientific Diagram
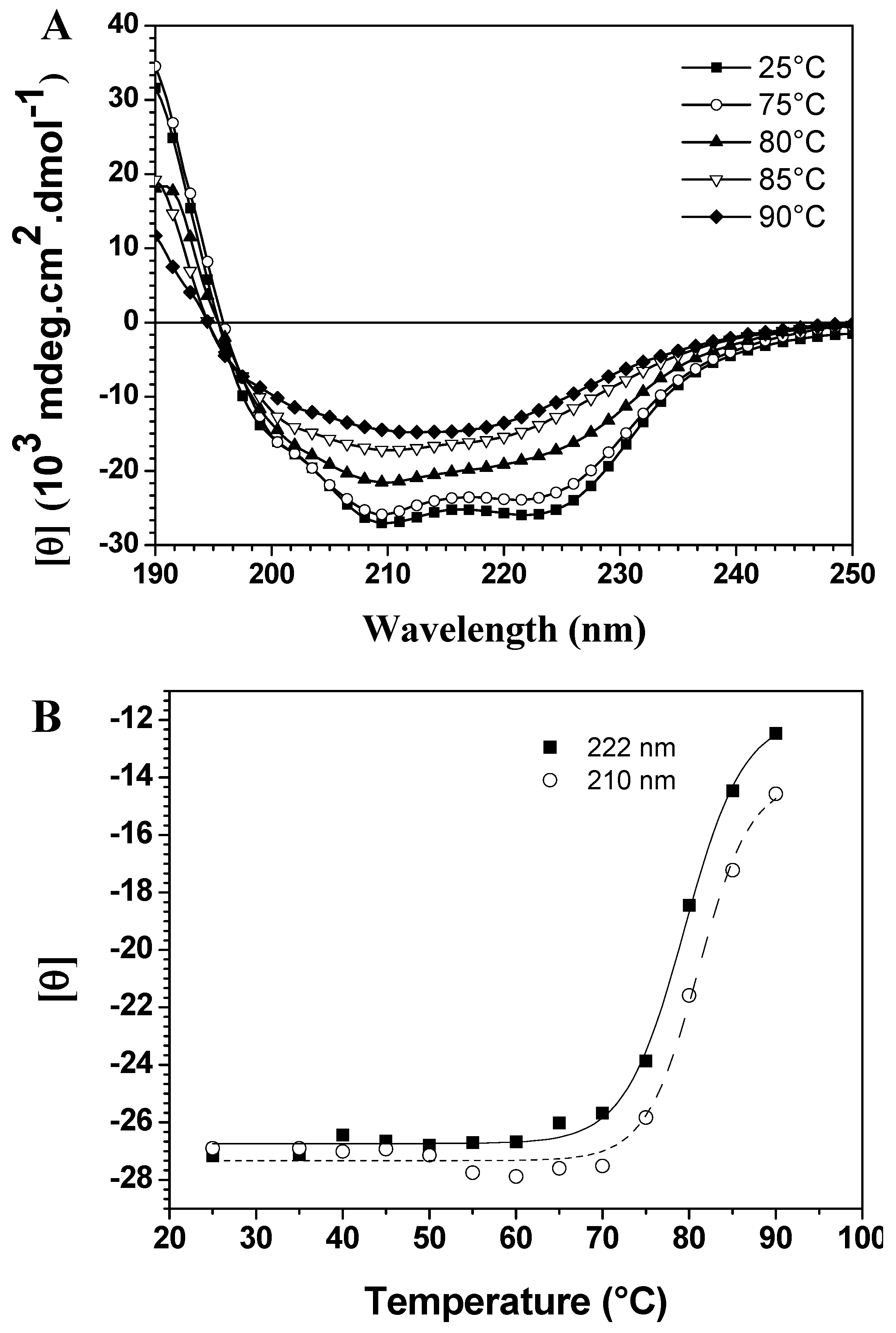


Molecules Free Full Text Unfolding Studies Of The Cysteine Protease Baupain A Papain Like Enzyme From Leaves Of Bauhinia Forficata Effect Of Ph Guanidine Hydrochloride And Temperature Html


Bg007a Guanidine Hcl 6m 바이오솔루션



Guanidine Hydrochloride Inhibits Mammalian Orthoreovirus Growth By Reversibly Blocking The Synthesis Of Double Stranded Rna Journal Of Virology



A New Approach To Study The Physical Stability Of Monoclonal Antibody Formulations Dilution From A Denaturant Journal Of Pharmaceutical Sciences



Imidacloprid Guanidine Hydrochloride Lgc Standards
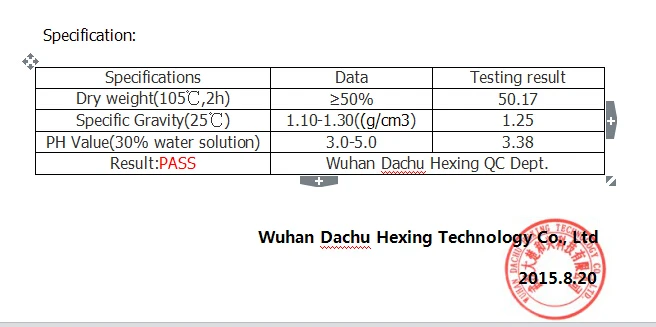


Poly Methylene Co Guanidine Hydrochloride Water Decolorant Buy Decolorant Product On Alibaba Com



Thermal Unfolding Of Lysozyme With Guanidine Hcl Measured With Dsc And Download Scientific Diagram



Usa1 Storage Of Nucleic Acid Google Patents



Figure 4 From Evidence For Residual Structure In Acid And Heat Denatured Proteins Semantic Scholar


Guanidinium Chloride Wikipedia



Figure 3 From Evidence For Residual Structure In Acid And Heat Denatured Proteins Semantic Scholar



Enhancing Colorimetric Loop Mediated Isothermal Amplification Speed And Sensitivity With Guanidine Chloride Biotechniques



Guanidine Hcl 6 Molar Gmp Denaturant Solution Guanidine Hydrochloride 6m Solution Bio Spectra



Exploring The Factors Affecting The Solubility Of Chitosan In Water Sogias 10 Macromolecular Chemistry And Physics Wiley Online Library



The Guanidine Thiocyanate High Edta Method For Total Microbial Rna Extraction From Severely Heavy Metal Contaminated Soils Pei 21 Microbial Biotechnology Wiley Online Library



Dual Effect Of Guanidine Hydrochloride On The Kinetics Of Aggregation Download Scientific Diagram
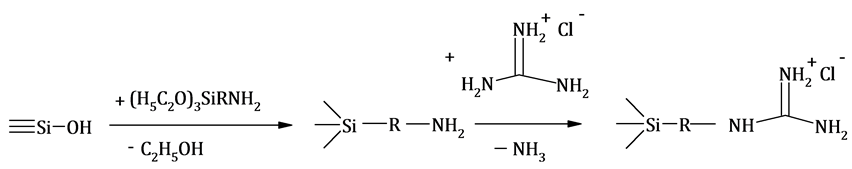


New Approach To Synthesis Of Silica With Chemically Bound Guanidine Hydrochloride For Preconcentration Of Metal Ions



Tryptophan Fluorescence Of Guanidine Hydrochloride Gdhcl Treated Download Scientific Diagram



Ph Corrections And Protein Ionization In Water Guanidinium Chloride Sciencedirect



Chlorhydrate De Guanidine Cristaux Incolores A Blancs Fisher Bioreagents Organonitrogen Compounds Organic Nitrogen Compounds Fisher Scientific


Plos One Loss Of Metal Ions Disulfide Reduction And Mutations Related To Familial Als Promote Formation Of Amyloid Like Aggregates From Superoxide Dismutase



No comments:
Post a Comment